The Medical Device Innovation Center (MIC) at University Medical Center Mainz supports industry, academia and clinics in developing innovative and state-of-the-art medical devices. We are here to assist you as a competent partner, translating your visions into reality. Leveraging clinical expertise and methodological excellence, we guide you through developing products and processes closely aligned with hospital requirements and in compliance with MDR regulations. Our aim is to efficiently navigate you through the development process. Furthermore, we provide support for Post-Market Surveillance (PMS) to ensure the long-term benefit and minimise risks associated with your medical devices.
As a Centre of Excellence for Medical Devices, we stand for close collaboration between industry, clinics, and research. We are committed to the safe and effective development of medical products to ensure the best possible healthcare for patients.
Background
The European Medical Device Regulation (MDR 2017/745) became effective on 25th May 2017, and becomes mandatory for all manufacturers from 26th May 2021. The aim of the regulation is to improve the safety of medical devices throughout the entire product lifecycle. Therefore, manufacturers are required to demonstrate the safety, performance and clinical benefit of their devices and to ensure transparent monitoring and traceability over the lifecycle.
The MDR poses substantive and financial challenges especially for small and medium-sized enterprises (SMEs), young companies, start-ups, but also scientists involved in the development of new, innovative medical devices. Companies are confronted with a number of additional requirements. Therefore, some consider the MDR as an obstacle to innovation – negatively impacting the medical device industry in Germany. According to the German Medical Technology Association (BVMed), the most relevant obstacles to medical technology development under the new MDR in Germany are the large number of regulatory requirements for the approval of a medical device as well as the obligation to provide comprehensive clinical data. This bottleneck to innovation in the development of medical devices must be solved in order to sustainably strengthen Germany as a location for medical device manufacturers.
In 2021, the MIC Mainz was founded to counteract this inhibition and to support small businesses, start-ups, but also established companies, lateral entrants and scientists in the development of innovative medical devices. The MIC contributes to advancing the development of new medical devices and improving the transparency as well as the traceability of medical devices with a broad range of services and cooperation partners. The aim of the MIC is to establish a close network between industry and clinics in order to support the development of new and innovative medical devices – thus strengthening the position of Germany as a center for medical devices in the long term. The MIC is funded by the Ministry of Economics, Transport, Agriculture and Viticulture (MWVLW) of Rhineland-Palatinate and is affiliated with the Interdisciplinary Centre for Clinical Studies (IZKS) of the University Medical Center Mainz.
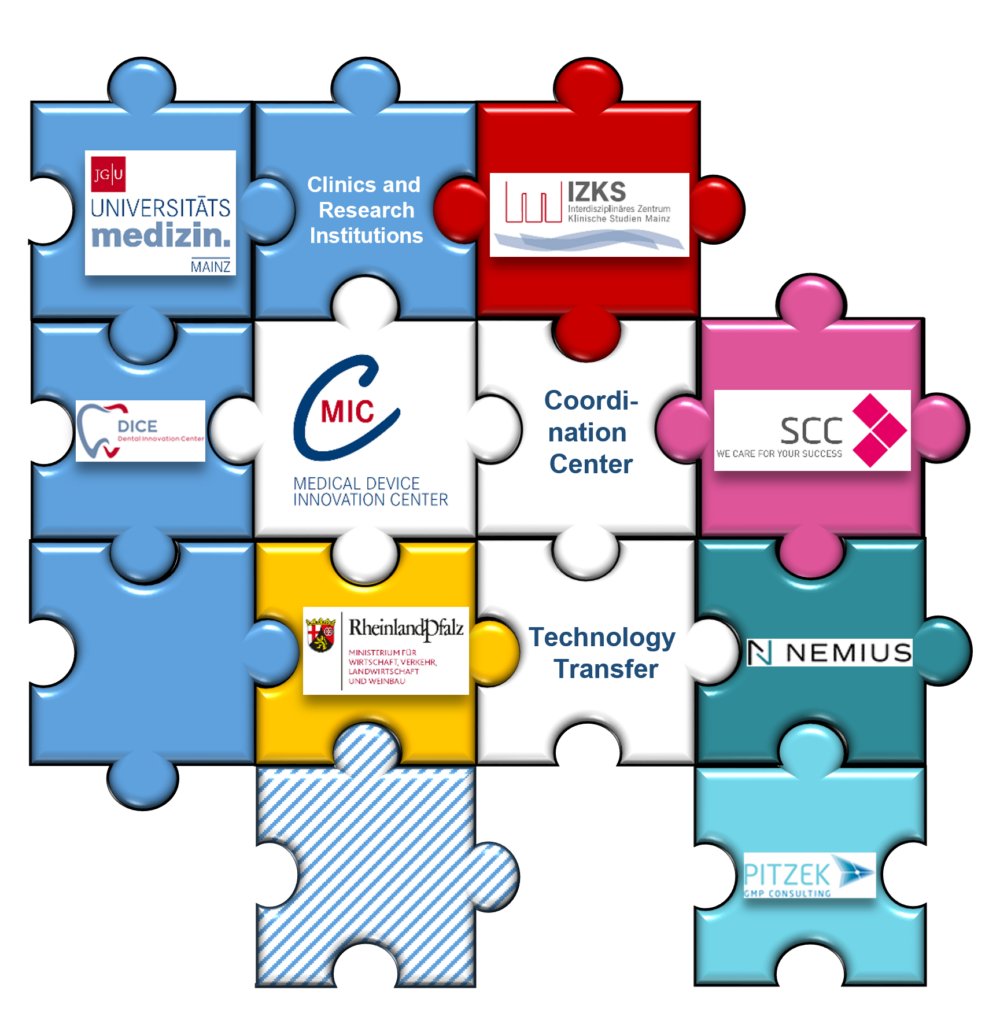
Structure and Organisation
The MIC understands itself as a central contact point and driving force within a large competence network. It provides both the necessary medical expertise as well as all the tools and skills required for the efficient development of new medical products.
About the University Medical Center Mainz
The University Medical Center of the Johannes Gutenberg University of Mainz is the only medical institution providing supramaximal care in Rhineland-Palatinate and is an internationally recognised scientific location. It comprises more than 60 clinics, institutes and departments working and treating together more than 300,000 inpatients and outpatients every year. The highly specialised patient care, clinical research and teaching are an inseparable unit at the Mainz University Medical Center. Around 3,300 medical and dental students as well as more than 600 specialists are trained in a wide variety of medical, commercial and technical professions. Additionally, the Mainz University Medical Center With about 8,600 employees is also one of the largest employers in the region and an important driver of growth and innovation.
The MIC contributes to the development of the Mainz University Medical Center, providing an innovation and competence center for medical devices. It supports companies in the development of innovative medical products by providing one central contact for all issues regarding clinical, technical as well as economical and regulatory.
About the IZKS Mainz
The Interdisziplinäre Center for Clinical Studies (IZKS) is a central research and teaching platform of the University Medical Center Mainz. The IZKS forms the backbone of the MIC competence network by providing support in the consultation, planning and implementation of clinical research projects in accordance with the German Medical Devices Act (MPDG). Experienced physicians, statisticians, computer scientists and natural scientists work together to ensure high efficiency and quality in the planning and execution of clinical research projects.
In addition, the IZKS also offers comprehensive consulting and supervision of clinical research projects in drug development according to AMG as well as training of study personnel according to AMG and medical device law in accordance with the current curricula. Further information can be found here: https://www.izks-mainz.de/veranstaltungen/
About the Ministry of Economy, Transport, Agriculture and Viticulture Rhineland-Palatinate
The Ministry of Economics, Transport, Agriculture and Viticulture (MWVLW) covers a wide range of disciplines, including economic and structural policy, cross-border cooperation, innovation promotion, viticulture, monitoring, agriculture, road construction, local public transport as well as basic mobility issues. Priority of the MWVLW is the strengthening of the economy in Rhineland-Palatinate , promoting economic growth and cerating new job opportunities To this end, various instruments of economic promotion and support are available.
The aim of the MWVLW is not only to support innovative ideas from start-ups, but also to promote the expansion of existing companies. Cornerstones of the MWVLW’s innovation strategy are the expansion of the application-oriented research and development infrastructure, the promotion of innovative start-ups, the support of innovations in individual companies, networks and clusters. The MIC Competence Center is one of these networks.
As a Rhineland-Palatinate Gold Partner, the MIC also has access to a strong network of around 4,500 researchers, universities, research institutions and company networks in Rhineland-Palatinate.
Technology Transfer
The technology transfer supports scientists and companies in creating innovative ideas, product development, funding- and IP consulting and company formation. Mainz University Medical Center is one of the leading partners of the Medical Start-up Alliance Rhein-Main (MSAR-M). In this alliance, start-up founders are supported, start-up teams are formed and accompanied to a long-term successful medical start-up. The open, regional exchange of medical start-ups takes place in the Medical start-up Entrepreneur Meeting (MUT). The University Hospital has prepared the ground for future developments so that this development will continue to be successful in the future.
The contact for technology transfer at Mainz University Hospital is Zahra Ghodratian.
Further Cooperation Partners
One of MIC’s external cooperation partners is the Scientific Consulting Company (SCC). The SCC is an international consulting company that independently supports manufacturers in solving scientific and strategic challenges – without any commercial ties to contract research organisations. In collaboration with the MIC, SCC’s Medical Devices Division provides regulatory and strategic support to medical device manufacturers. In this context, the SCC is also able to perform clinical evaluation of class I to III medical devices.
For more information on the collaboration between the MIC and the SCC, please see our joint flyer:
A more recent addition to our network is Pitzek GMP Consulting. For over a decade, Pitzek GMP Consulting has been synonymous with comprehensive consultancy services for the pharmaceutical, biotechnology and food industries. With offices in the Rhine-Neckar region, Berlin and Vienna, the company serves clients of various sizes. The experts at Pitzek GMP Consulting bring extensive experience from international projects and offer expertise in implementing quality management systems according to DIN ISO 9001/13485 and GxP guidelines. They also specialise in analysing and developing hygiene concepts for cleanrooms, including aspects such as cleanroom cleaning, behaviour and logistics. Moreover, their range of services includes the qualification and validation of production equipment, engineering of production facilities, including feasibility studies and communication with manufacturers, as well as automation and computer system validation. Ongoing staff training ensures that their professionals are always up to date with the latest technologies, project management and legal requirements.
A recent addition to the MIC network is NEMIUS Group GmbH, a specialised consultancy for the medical device and in vitro diagnostics (IVD) sectors. The company supports manufacturers in meeting regulatory requirements and optimising their business processes. Its core services include the development and implementation of management tools as well as process optimisation to ensure the quality and safety of medical devices and IVDs. In close collaboration with MIC, NEMIUS Group GmbH brings its expertise to advance innovative approaches in the development and optimisation of medical devices and IVDs. This new partnership enables us to further expand our network and provide even more targeted support to meet the needs of the MedTech and diagnostics sectors.
Vision
The MIC aims to strengthen and support the medical device industry in Rhineland-Palatinate and Germany. We continuously expand our range of services and strive to become the leading partner for Digital Health Applications (DiGA) and research projects related to the new regulation on In-vitro Diagnostics Devices (IVDR). Additionally, the MIC is dedicated to driving the further development of the Rhineland-Palatinate competence network as a central point of contact for medical devices.
We are open to future cooperations and research projects. For further information as well as a personal contact you can reach us here.
We look forward to meeting you!
