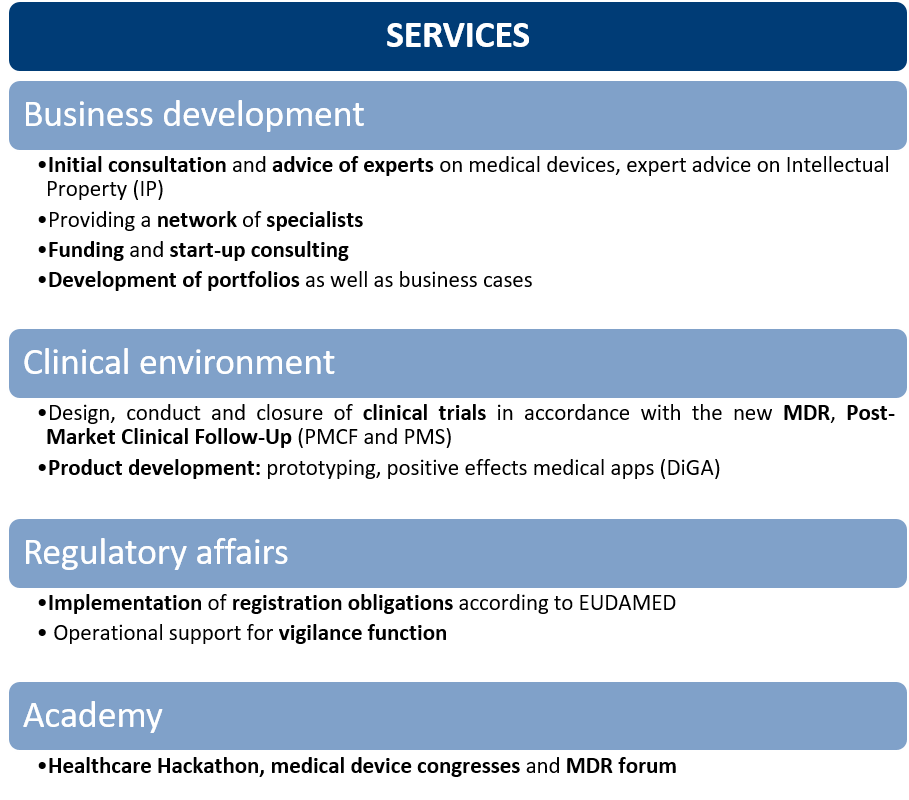
Our comprehensive range of services covers various areas. In the field of Business Development, we provide you with consulting and expertise on medical devices. We also support you with intellectual property (IP) matters and connect you with valuable contacts to industry experts.
Another focus is on the clinical environment. We assist you in the planning, execution and completion of clinical studies in accordance with the MDR. Additionally, we accompany you in the development and evaluation of medical apps (DiGA) and offer support in product development and prototyping.
In the area of Regulatory Affairs, we assist you in implementing the registration requirements according to EUDAMED. Our experts provide operational support in fulfilling your vigilance function.
Furthermore, we actively contribute to the development of the University Medical Center Mainz as an innovation and competence center for medical device research and development. We are your central point of contact in the Rhine-Main region and provide you with a strong network of collaboration partners.
Contact us to collaborate on your medical innovation.